Safety and tolerability
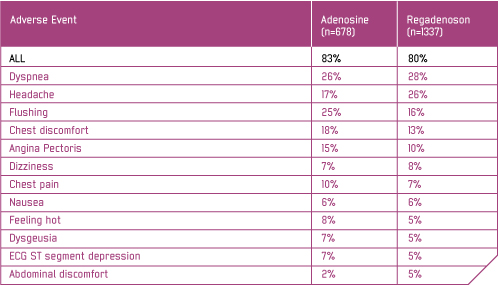 Adverse events with incidence ≥ 5% In ADVANCE MPI 1 and ADVANCE MPI 2, the following pre-specified safety and tolerability endpoints comparing Rapiscan to adenosine achieved statistical significance: (1) a summed score of both the presence and severity of the symptom groups of flushing, chest pain, and dyspnoea was lower with Rapiscan (0.9 ± 0.03) than with adenosine (1.3 ± 0.05); and (2) the symptom groups of flushing (21% vs 32%), chest pain (28% vs 40%), and 'throat, neck or jaw pain' (7% vs 13%) were less frequent with Rapiscan; the incidence of headache (25% vs 16%) was more frequent with Rapiscan. In clinical trials first degree AV block (PR prolongation > 220 msec) developed in 3% of patients within 2 hours of Rapiscan administration; transient second-degree AV block with one dropped beat was observed in one patient receiving Rapiscan. In post-marketing experience, third degree heart block and asystole have been reported within minutes of Rapiscan administration. In the ADVANCE MPI Trials, continuous electrocardiographic monitoring shows Rapiscan to be associated with less AV block than adenosine. References
|